LCRC Faculty Collaborate On Late-Stage Breast Cancer Research
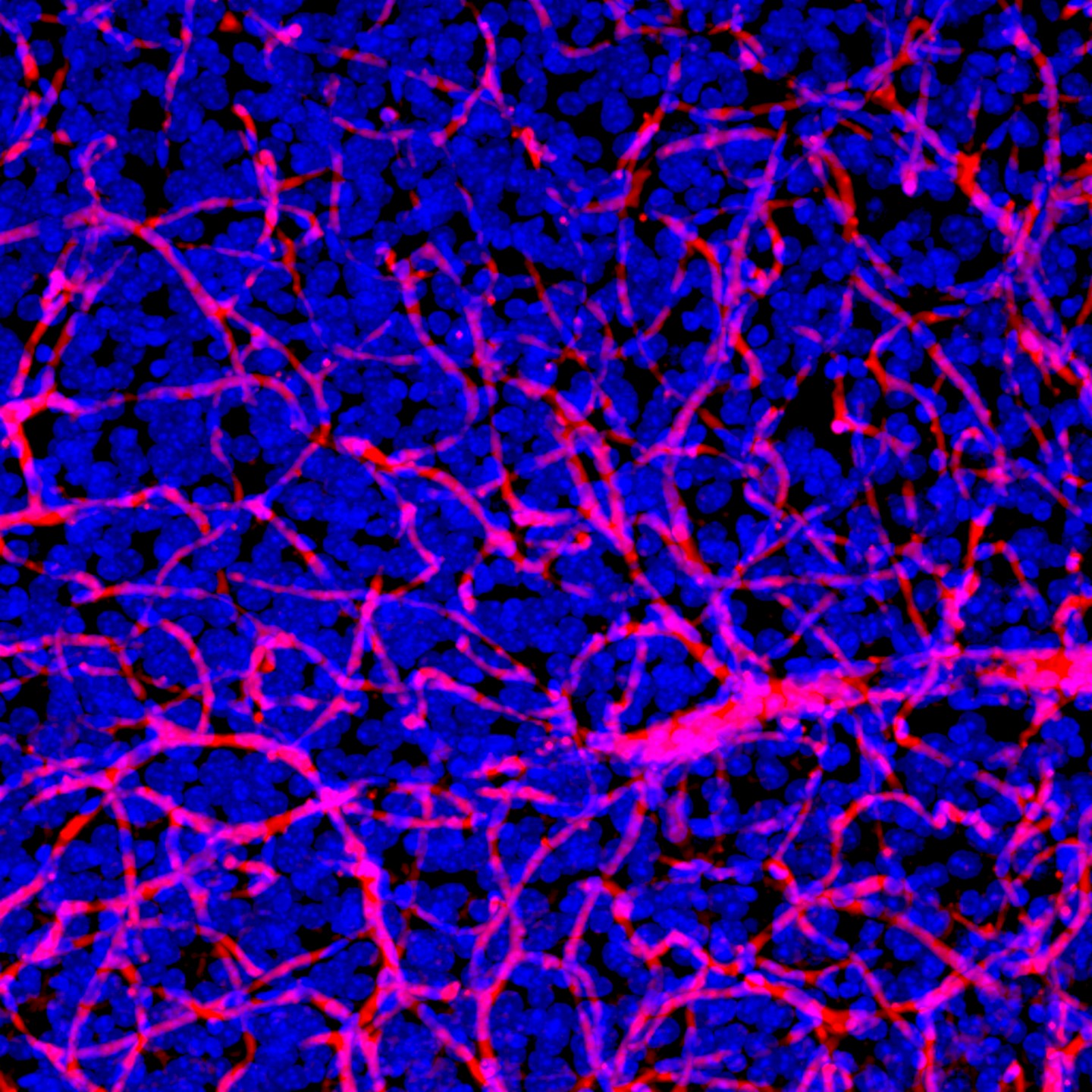
Above: Dense network of blood vessels (red) and nuclei (blue) obtained from animal brain tissue that was optically cleared to look deeper into the tissue than otherwise possible. The brain’s blood vessels are nearly impermeable allowing only the passage of key nutrients while blocking harmful substances. The blood-brain barrier also prevents most therapeutics from entering the brain. photo credit: CA Institute of Technology
Once Stage 4, or breast cancer brain metastasis, (BCBM), reaches the brain, it becomes extremely challenging to treat. A second hurdle to overcome is that BCBM typically is diagnosed after treatment with frontline therapies, so the BCBM cells may already be drug-resistant. Third, all treatments must get pass the blood brain barrier, which serves as a protective filter for the brain and is impenetrable for many cancer-fighting drugs.

Tulane Professor and LCRC faculty member Tiffany Seagroves, MBA, PhD, PMP, is using animal models of BCBM to test the safety and efficacy of the latest generation of tubulin-targeting compounds in a partnership with The University of Tennessee Health Science Center (UTHSC) in Memphis, TN. Several FDA-approved drugs target tubulin, such as paclitaxel (Taxol), but up to a third of patients taking “taxane”-class of drugs will develop resistance through multiple mechanisms. Those drug-resistant pathways are typically avoided when compounds targeting the colchicine-binding site on tubulin are used, known as colchicine-binding site inhibitors (CBSIs). In addition, several CBSIs cross the blood-brain barrier and therefore have the potential to treat brain metastases from breast, lung, or melanoma cancers, or to treat primary gliomas that originate in the brain. Dr. Seagroves also uses these same animal models to study how the creatine kinase metabolic pathway promotes metastasis.
Dr. Seagroves co-leads the Louisiana Cancer Research Center’s Cancer Biology Program with Krzysztof Reiss, PhD, Professor of Medicine at the LSU LCMC Health Cancer Center.

When Dr. Seagroves joined Tulane and the LCRC faculty in 2024, she learned about Dr. Reiss’s research targeting glioblastoma (brain tumor) cellular metabolism, or the pathways tumor cells use to produce energy. Tumor cells often rely on specific metabolic pathways to survive, and these can be weak points ideal for drug targeting. “We are looking at energy metabolism and trying to intervene with the way tumor cells produce energy. We are also trying to modify the structure of compounds that allow them to cross the blood-brain barrier,” Dr. Reiss explained.
“When I realized [Dr. Reiss] had compounds that also crossed the blood-brain barrier and also targeted cellular metabolism, but through a different pathway, I thought, ‘what if we put them together?’,” Dr. Seagroves said. “Hopefully, we’ll find some synergy there.”
Dr. Reiss agreed. “We are working to develop combinational therapy targeting distinct pathways that breast cancers and gliomas rely on for survival using animal models and three-dimensional organoid cultures,” Dr. Reiss explained. “We are also using animal models to grow those glioma or breast cancer cells in the brain and trying to use our compounds to penetrate the blood-brain barrier and inhibit their growth.” Dr. Seagroves added, “We will not only share compounds, but we will leverage our combined expertise and research models in breast cancer and glioblastoma to treat cancer lesions in the brain.”
Still in its early stages, preliminary evidence is promising. The collaboration represents an important effort toward developing much-needed therapies for drug-resistant brain tumors.